Apolipoprotein E (ApoE) Isoforms & Macrophage Exosomes
Apolipoprotein E (ApoE) Isoforms & Macrophage Exosomes: Impact on Immunometabolism, Atherosclerosis and Neurodegeneration
Our team has built on a rich legacy of ApoE-related research in lipoprotein metabolism, inflammation and atherosclerosis. Recently, our findings uncovered that ApoE serves as a natural immune checkpoint in atherosclerosis by restricting myeloid cell activation through an intrinsic capacity to modulate cellular microRNA levels (PMID: 25904598).
We uncovered that an ApoE/microRNA axis regulates inflammation in the cardiovascular system by controlling bioenergetic activity and thereby immunometabolism in myeloid cell (PMID: 37824329).
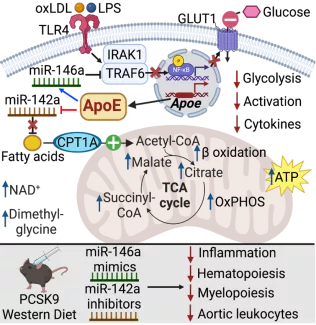
Key Findings
- ApoE increases miR-146a in myeloid cells & HSPC, reducing glucose uptake & glycolysis
- By reducing miR-142a, ApoE drives fatty acid oxidation to improve mitochondrial metabolism
- ApoE thereby regulates immunometabolism to control hematopoiesis & inflammation and atherosclerosis in hyperlipidemia
MiR-146a mimics and miR-142a inhibitors can serve to substitute for ApoE in controlling cardiometabolic inflammation and atherosclerosis in hyperlipidemia
Building on our findings, we tested whether ApoE/microRNA axes can serve to control inflammation and atherosclerosis at a distance via cell-derived extracellular vesicles (EVs) also known as exosomes.
In a follow up study, we uncovered that beyond stimulating cellular lipid efflux, macrophage ApoE expression results in the production of “ApoE-rich” exosomes that display a potent capacity to communicate immunometabolic activities to recipient myeloid cells and T lymphocytes (PMID: 37593979).
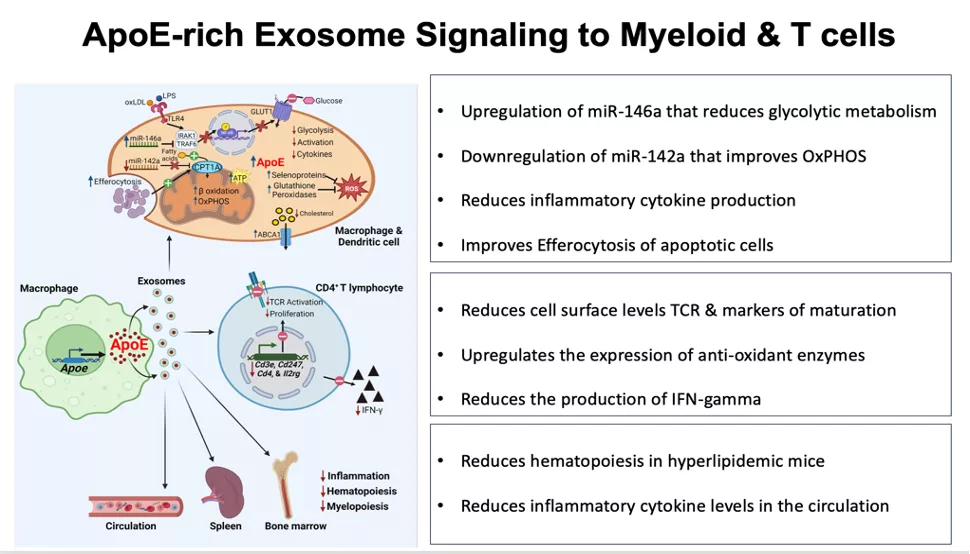
Our findings unveil a previously unsuspected role for cellular ApoE expression in controlling the intercellular signaling properties of secreted EVs including exosomes. Furthermore, this mode of signaling by ApoE sheds new light to understand the capacity for ApoE to control T cell activity and thereby adaptive immunity in atherosclerosis
ApoE isoform-Specific Control of Immunometabolism: Impact on Extracellular Vesicles
Ongoing studies test the hypothesis that biochemical and structural differences that distinguish human ApoE isoform activity in lipoprotein metabolism also impact microRNA-controlled axes in cellular biogenetic metabolism and immunometabolic activity in monocytes, macrophages and their hematopoietic progenitor cells (HSPC) in the bone marrow and the spleen.
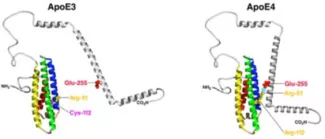
Domain Interaction in ApoE4 contributes to its altered lipoprotein distribution preference in the circulation and lipidation status in cells (PMID: 8702576). We are testing if this also alters microRNA controlled axes in bioenergetic metabolism
We are testing the hypothesis that ApoE4 is less potent than ApoE3 in augmenting mitochondrial metabolism to drive OxPHOS and thereby atheroprotective properties including myelopoiesis and efferocytosis.
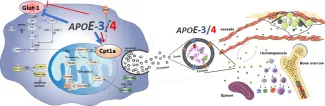
We are also testing the idea that ApoE4-exosomes are enriched with sets of microRNA and cellular metabolites that contribute to accelerate cardiometabolic disease including atherosclerosis by communicating inflammatory signaling to HSPC as well as to mature myeloid cells, T cells, and Tregs.
Through collaborative efforts we are testing whether ApoE4-exosomes contribute to neurodegenerative disorders along with impaired cognitive recovery following a stroke or traumatic brain injury.
